Have you ever wondered how surgical masks are different from general masks?!! and what is ASTM?
Surgical masks must meet the standards set by the Food and Drug Administration (FDA), which are consistent with the industrial product standards of the Ministry of Industry and can be compared with international standards such as the American Society of Testing standards and Materials (ASTM), audited by the National Institute for Occupational Safety and Health (NIOSH). It is divided into 3 levels of surgical masks: Low Barrier, Medium Barrier and High Barrier according to the physical properties of the mask as follows:
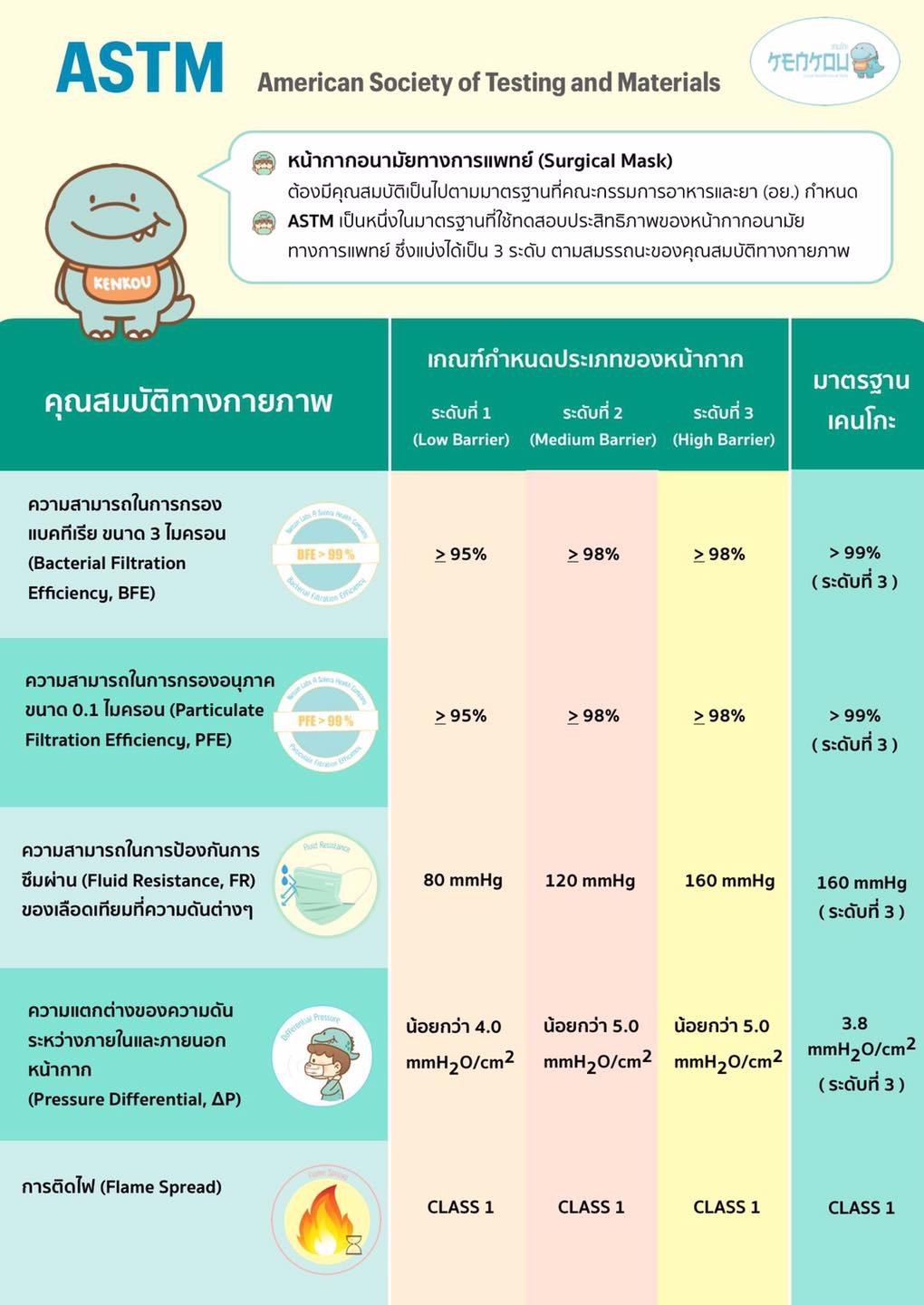
Bacterial Filtration Efficiency: BFE
Bacterial filtration efficiency is a value that indicates the ability of a mask to filter out particles of bacteria and other microorganisms with a mean particle size (MPS) of 3 ± 0.3 microns.
Viral Filtration Efficiency: VFE
Virus filtration efficiency is a value that indicates the ability of a mask to filter viral particles with a mean particle size (MPS) of 3 ± 0.3 microns.
Fluid Resistance
The ability of Fluid Resistance from the outer layer to inner layer of mask using artificial blood for permeability testing at 80 mmHg (Low Barrier), 120 mmHg (Medium Barrier), and 160 mmHg (High Barrier).
Differential Pressure: Delta P
Differential Pressure measure of the air resistance of the mask to see the ease of breathing. The lower the resistance, the easier it is to breathe. As a standard, the value must be less than 5 mm H2O/cm2.
Flammability
There are 3 levels of flammability testing assessed by burning speed and flame (Class 1, 2, 3). Class 1 will be the best (Burning slowly and igniting flames within a short distance).
Bacterial filtration efficiency is a value that indicates the ability of a mask to filter out particles of bacteria and other microorganisms with a mean particle size (MPS) of 3 ± 0.3 microns.
Viral Filtration Efficiency: VFE
Virus filtration efficiency is a value that indicates the ability of a mask to filter viral particles with a mean particle size (MPS) of 3 ± 0.3 microns.
Fluid Resistance
The ability of Fluid Resistance from the outer layer to inner layer of mask using artificial blood for permeability testing at 80 mmHg (Low Barrier), 120 mmHg (Medium Barrier), and 160 mmHg (High Barrier).
Differential Pressure: Delta P
Differential Pressure measure of the air resistance of the mask to see the ease of breathing. The lower the resistance, the easier it is to breathe. As a standard, the value must be less than 5 mm H2O/cm2.
Flammability
There are 3 levels of flammability testing assessed by burning speed and flame (Class 1, 2, 3). Class 1 will be the best (Burning slowly and igniting flames within a short distance).



